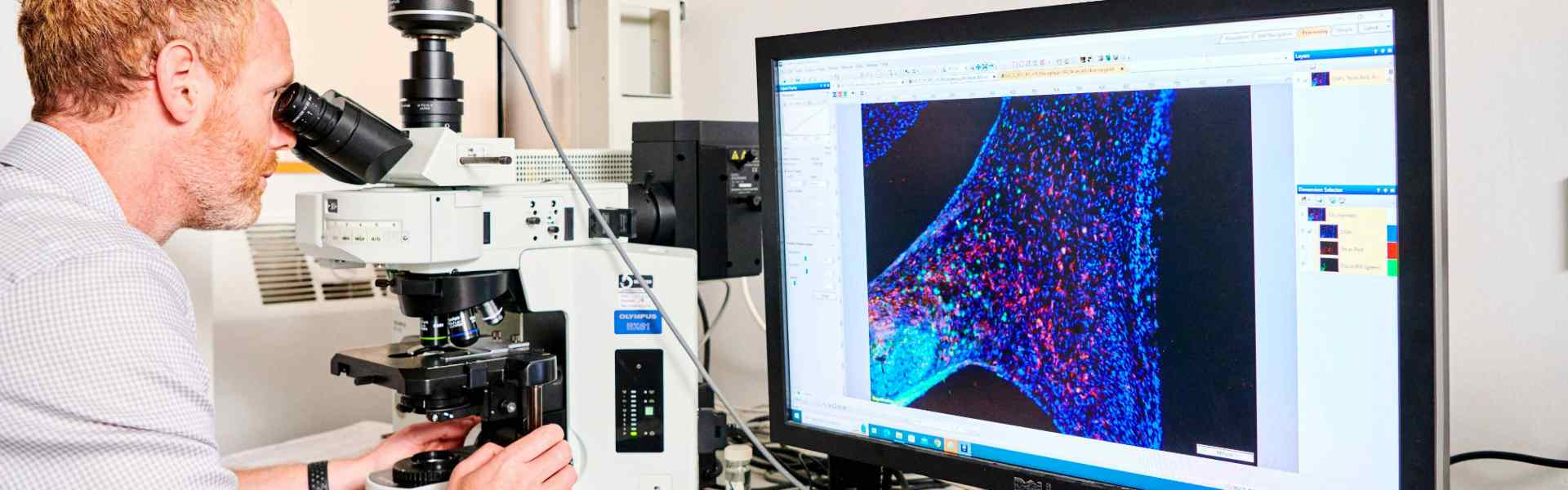
Oral Microbiology
Our research aims to understand the basic biology of microorganisms that are associated with human health and disease, including mechanisms of resistance to antimicrobial agents. We aim to develop more effective approaches to prevent or treat microbial-driven oral diseases such as dental caries (tooth decay) or periodontitis (gum disease).
.png)
Dr Chang's research aims, current projects and partners...
Our research aims to understand how do bacteria sense and respond to their surrounding environment and stresses by genetic and genomic approaches with advanced sequencing and microscopic techniques. We are also interested in harnessing bacteria to produce useful metabolites for therapeutical or biotechnological purposes.
Current projects
- Characterisation of nano-micelles for next generation antimicrobial agents
- Unravelling the complex metabolic interplay between Pseudomonas aeruginosa and host microenvironment in response to last-line antibiotics
- Characterising genetic regulations and heterogeneous phenotypes of antibiotic persistence in Pseudomonas aeruginosa
- Investigating the microbial influence on the metabolic imbalance in pulpitis
- Investigating the immunomodulatory mechanisms of oral bacteria causing inflammation on mucosal surfaces
Works with
Professor Jakubovics's research aims, current projects and partners...
Our group focuses on the impact of the microbiome on oral health, particularly aiming to understand the processes involved in the formation of dental biofilms. We aim to develop new approaches to control biofilm formation and biofilm-associated diseases in the mouth and elsewhere in the body.
Current projects
- Importance of bioaerosols for infection control in dentistry.
- Microbiome of dental calculus
- Ultrasmall bacteria in the oral microbiome
- Metabolic interactions in oral biofilms
- Characterising the oral biofilm matrix
Works with
Dr Da Silva Dantas's research aims, current projects and partners...
Replicative ageing, pathogenicity and drug resistance in Candida spp.
My laboratory is interested in determining the mechanist basis used by human fungal pathogens to survive environmental stress encountered in the host. We use a range of molecular, cellular and biochemical assays to determine the mechanistic basis on how Candida albicans overcome extreme stresses posed in the mammalian niche.
Current projects
- Identification of molecular mechanisms driving antifungal drug resistance in aged Candida populations. Funded by The Royal Society.
- Extrachromosomal DNA as a mediator of rapid, transient genetic adaptation in fungal pathogens. Funded by the Welcome Trust (collaboration with the Babraham Institute).
- Imaging biofilms to screen for novel antimicrobial compounds. Funded by an Industrial partnership with Haleon.
PhD and Masters projects:
- Dissecting the molecular mechanisms driving antifungal drug resistance and immune evasion in an aged Candida albicans population – MRC Dimen Studentship
- Targeting redox sensitive antioxidants to combat antifungal resistance – MRC Dimen Studentship (https://www.findaphd.com/phds/project/mrc-dimen-doctoral-training-partnership-targeting-thioredoxin-reductase-to-combat-antifungal-resistance-in-candida-spp/?p189446).
- Survival of the Oldest: Mitochondrial Remodelling and Antifungal Resistance in pathogenic yeast.
- How does age-associated stress tolerance in C. albicans promotes colonization and drives disease in a C. elegans model of infection?
- Endodontic Infections: The Overlooked Reservoir of Antifungal Resistance?
- Glucose-Dependent Survival of Candida albicans in Root Canal Treatments: Insights into Diabetes-Associated Endodontic Infections
Current lab members:
Dr Alina Goldberg Cavalleri (PDRA on Welcome Project)
Eloise Mitchell (MRC Dimen PhD student)
Technician (TBC – Imaging Biofilms).
Works with
- Royal Society
- Medical Research Council
- Biotechnology and Biological Sciences Research Council (BBSRC)
- Wellcome Trust
- British Mycological Society (BMS)
- Industry
Follow Dr Da Silva Dantas on LinkedIn